The problem
Aging is the main factor contributing towards both Parkinson's (PD) and Alzheimer's (AD) diseases. These chronic diseases are incurable and their disabling effects may continue for years or even decades.
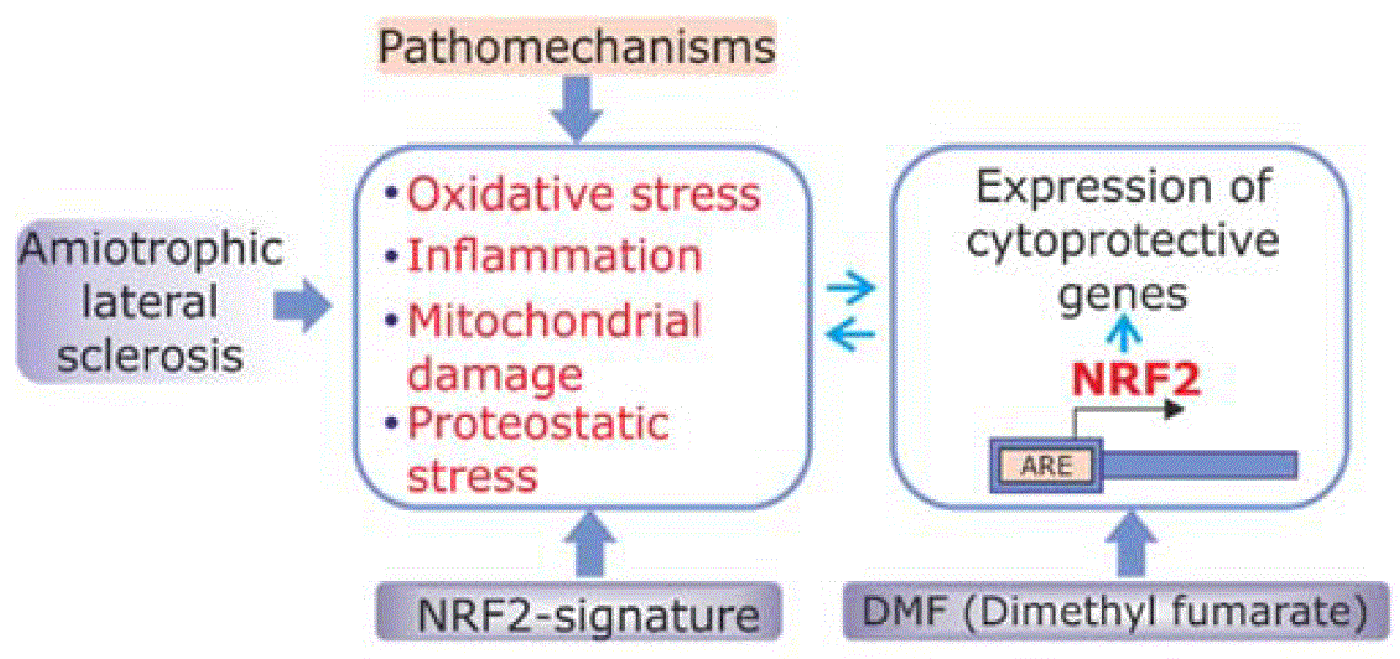
Studies on animal models of AD and PD and on human postmortem brain tissues, indicate that many pathological changes in the brain derive from a network of local stresses, like oxidative stress, tightly connected to inflammatory and proteotoxic stresses. Local stressful conditions are probably challenged by pathologically modified proteins, and, through a vicious cycle, may further trigger alteration of key molecules.
Our team has been studying protective mechanisms used to maintain homeostatic respones and how these mechanims coudl be targeted pharmacologically to provide superior defense.
Our approach
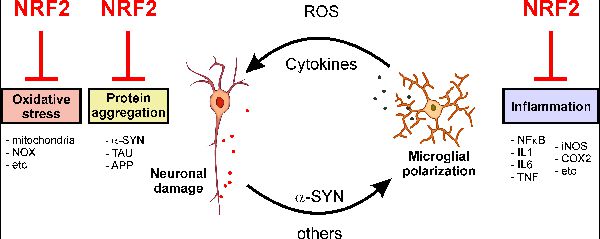
We are currently studying the role of transcription factor NRF2 in protection against stimuli that induce neurodegeneration.
NRF2 is a protein that regulates the expression of about 250 genes. These genes possess the antioxidant response element (ARE) in their promoters. The genes participate in adaptive responses to oxidative, inflammatory and proteotoxic stress and in the regulation of enzymes involved in biotransformation and glutathione metabolism.
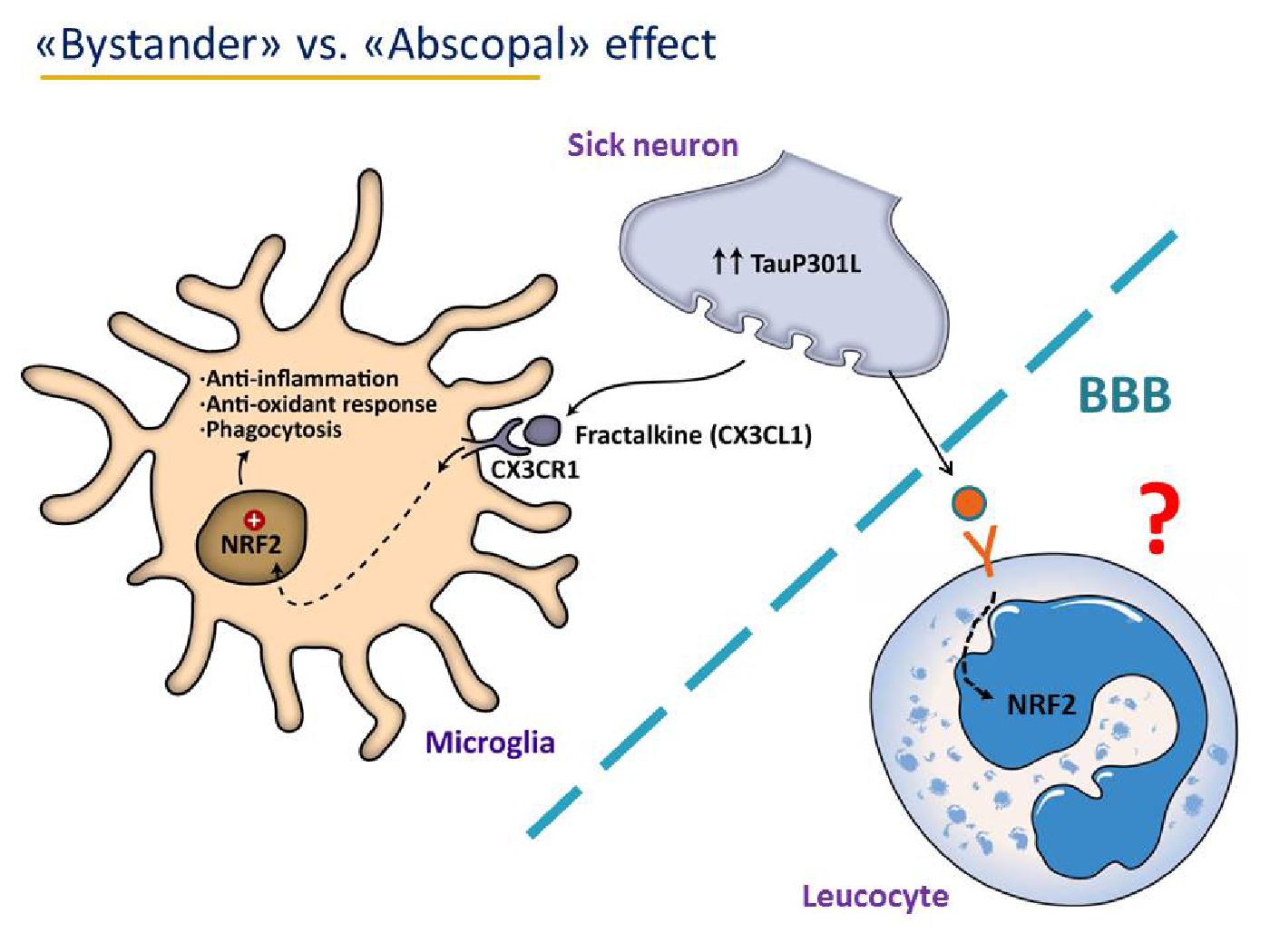
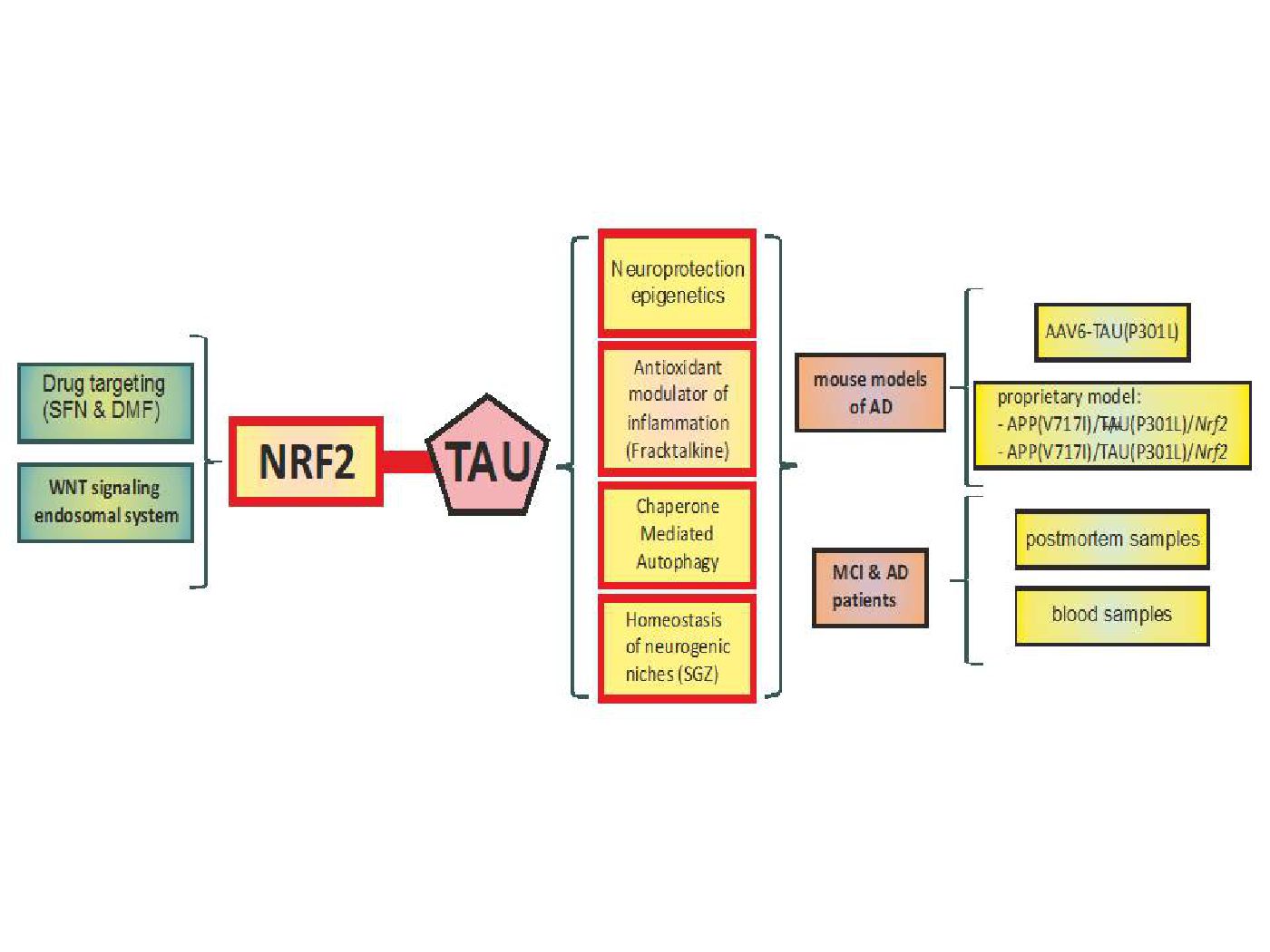
Using genetically modified rodent models as well as pharmacological approaches, we are studying the contribution of this transcription factor to the protection against oxidative damage and neuroinflammation in toxic (MPTP and 6-OHDA) and genetic (alpha-synuclein) models of Parkinson's disease and in transgenic mice possessing amyloidopathy (APPV717I) and tauopathy (TauP301L) , which are characteristic of Alzheimer's disease.

Objectives
• Generation of knowledge: Understanding the mechanisms that regulate NRF2 is fundamental to determine its physiological role and its pathological alterations as well as to design new pharmacological strategies. We are currently studying the regulation of NRF2 by signaling pathways. We have already described its regulation by the GSK-3/beta-TrCP pathway. We are now analyzing its participation in cell signaling by primary cilium and proliferative stimuli.
• Low-grade chronic inflammation is a key element of neurodegenerative diseases. We are studying the crosstalk between NF-kB and NRF2, key elements in the pro and anti-inflammatory phenotypes of microglia.
• Applicability: in collaboration with several companies, we are looking for novel mechanisms of regulation of NRF2 in the brain that could serve to reinforce its activity against neurotoxic stimuli. In preclinical models of Parkinson's disease, we are focusing on repurposing of dimethyl fumarate, a compound already used in clinical practice for multiple sclerosis.